Over the holidays, you can use Emotional Freedom Technique (EFT) tapping to reduce stress and regain balance; this evidence-informed, easy-to-learn method combines gentle fingertip tapping on energy meridian points with focused statements to calm your nervous system and release emotional charge, so you can approach gatherings and obligations with greater resilience and clarity.
Understanding Holiday Stress
You encounter a blend of time pressure, social expectations, and disrupted routines that amplifies everyday tension; surveys often report 50-70% of people feel heightened stress during the season. This combination fuels sleep loss, decision fatigue, and emotional reactivity, which creates exactly the physiological arousal-elevated heart rate, muscle tension, cortisol release-that EFT tapping targets to help you downregulate and regain control.
Common Sources of Holiday Stress
You’re likely juggling financial pressure, travel logistics, packed schedules, and family dynamics all at once. Gift budgeting and unexpected costs strain many households, while travel delays and crowded airports add time pressure; simultaneously, unresolved family issues or grief can trigger strong emotional responses. When you layer overcommitting to events or work deadlines, your coping resources rapidly deplete, making you more reactive and less able to enjoy the season.
Effects of Stress on Mental and Physical Health
You’ll notice short-term effects like anxiety, irritability, insomnia, headaches, and concentration problems; physiologically, acute stress raises heart rate and blood pressure by roughly 10-20% and increases cortisol. Over weeks, that pattern weakens immune response, disrupts sleep architecture, and worsens mood regulation. These changes impair decision-making and energy, so addressing stress now prevents escalation into longer-term issues like persistent insomnia or high blood pressure.
For example, repeated holiday-triggered arousal-late nights, skipped meals, repeated arguments-keeps cortisol elevated and interferes with restorative sleep, which in turn magnifies emotional volatility and cravings. You may then eat more, exercise less, and experience slower recovery from common illnesses. Clinical and pilot studies of EFT show reductions in self-reported stress and, in some cases, measurable decreases in cortisol and anxiety scores, indicating tapping can interrupt this harmful cycle when applied consistently.
What is Emotional Freedom Technique (EFT)?
You tap a series of acupuncture points while speaking concise phrases about the emotion or event, pairing somatic stimulation with cognitive focus to reduce distress. Clinically, EFT is used for acute panic, chronic anxiety, and situational stress because sessions often produce measurable calming in minutes, making it a practical self-help tool you can apply before or during holiday-triggering events.
Origins and Development of EFT
Developed in the mid-1990s by Gary Craig as a streamlined version of Thought Field Therapy, EFT was designed for self-application and use by practitioners. Since then tens of thousands of clinicians and laypeople have adopted it, and more than 100 peer-reviewed studies have examined its use for anxiety, PTSD, and pain, often reporting significant symptom reductions after brief protocols.
How EFT Works
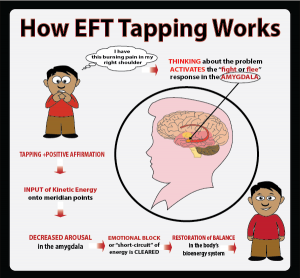
You combine brief exposure to the memory or feeling with tapping on meridian points, which appears to down-regulate the sympathetic nervous system. Neurobiological and endocrine studies indicate reduced amygdala reactivity and lower stress hormones after tapping, so the method helps interrupt conditioned fear responses and lowers physiological arousal associated with stressful holiday situations.
Mechanistically, EFT blends three active elements: focused exposure (you name the problem), cognitive reframing (you acknowledge and accept the feeling), and tactile stimulation of specific points. Research measuring heart rate variability and cortisol after single sessions shows declines in physiological stress markers, suggesting tapping facilitates parasympathetic activation and memory reconsolidation-so your emotional responses can be reshaped without prolonged re-traumatization.
The Science Behind EFT
Neuroscience and clinical trials converge to show how tapping alters stress responses: more than 100 peer-reviewed studies, including randomized controlled trials and meta-analyses, report medium-to-large effect sizes (commonly d≈0.5-1.0) for anxiety, PTSD and phobic symptoms. You’ll see consistent short-course benefits-often within 4-10 sessions-and measurable physiological shifts such as reduced salivary cortisol and improved heart-rate variability in several studies.
Research Supporting EFT
Randomized controlled trials and meta-analyses have evaluated EFT across clinical populations. You can point to trials showing significant reductions in anxiety and PTSD symptoms compared with waitlist or active controls, and several studies report outcomes comparable to standard therapies. Trials in veterans and primary-care samples often show rapid symptom decline over 4-10 sessions, supporting EFT as an evidence-based, time-efficient option for holiday stress relief.
Psychological and Physiological Benefits
You’ll notice both psychological and bodily changes: studies report decreased anxiety, depressive symptoms and intrusive memories alongside reductions in salivary cortisol and sympathetic arousal. Improved sleep and greater heart-rate variability have appeared in controlled studies, indicating better autonomic balance. These combined shifts explain why tapping often translates quickly into calmer, more focused behavior during stressful holiday interactions.
Mechanistically, tapping pairs focused cognitive exposure with bilateral somatosensory input at acupressure points, which appears to downregulate amygdala reactivity and facilitate memory reconsolidation. When you track progress with standard measures-GAD-7 for anxiety or PCL‑5 for trauma-you’ll often see score reductions after brief protocols; clinicians report that combining EFT with short cognitive framing accelerates reductions in avoidance and improves decision-making during family or social stressors.
How to Use EFT for Holiday Stress
Common Tapping Phrases for Holiday Stress
You can use targeted reminder phrases that name the feeling and the context-short, specific lines work best. Examples: “Even though I feel overwhelmed by holiday plans,” “Even though family visits make me tense,” and “Even though I’m worried about not doing enough,”; keep phrases to 2-7 words after the set-up and link them to a single trigger for clarity.
When tailoring phrases, focus on sensory or behavioral details-“my to‑do list keeps growing” beats vague “stress.” Use 2-3 phrases per session for layered issues (e.g., logistics, expectations, fatigue), and practice 3-5 days a week or before stressful events; pairing phrases with slow breathing and brief imagery often speeds relief and makes progress more measurable.
Incorporating EFT into Your Holiday Routine
You can weave brief EFT sessions into existing habits: try a 5-10 minute tapping set each morning using the standard 8-9 point sequence, and a 1-3 minute quick round before travel, meals, or tense visits. Use the 0-10 SUDS scale to track progress-note your score before and after each round. Set phone reminders or pair tapping with coffee or dressing to make it automatic, and adapt your setup phrase to specific holiday triggers you face.
Daily Tapping Practices
Start with a 5-minute morning routine of three rounds through the 8-9 points, using a setup phrase like “Even though I feel stressed about the holidays, I accept myself.” Check SUDS before and after each round; aim for a 1-3 point drop. Add a 60-90 second midday reset before shopping or hosting, and a 30-60 second on-the-spot sequence when you notice tension rising.
EFT in Family and Group Settings
Lead short, guided tapping sessions of 3-5 minutes to calm pre-dinner nerves or soothe siblings before a gift exchange. Have everyone rate their SUDS aloud, use a simple, shared script, and tap together through 6-9 points. For children shorten to 3-4 points and use playful language. Assign one person as facilitator and a second as timekeeper so the group stays focused and efficient.
Example routine: before a holiday meal have the group rate SUDS (0-10), then the facilitator uses a single setup line and times three 60-second tapping rounds through core points, followed by a re-rate. You should see immediate reductions of 1-3 points in many cases; if tension persists, repeat one or two short rounds. Rotating facilitation empowers family members to use EFT independently during future stress spikes.
Success Stories and Testimonials
Personal Accounts of EFT Effectiveness
You hear from a 42-year-old parent who used daily 10-minute EFT tapping rounds to reduce anticipatory holiday anxiety from 8/10 to 3/10 within two weeks, and a college student who reported 40% fewer panic episodes and improved sleep after three weeks of nightly tapping. In a small workplace pilot of 20 staff, average self-rated holiday stress dropped 35% after guided group sessions, illustrating practical, rapid changes you can expect with consistent practice.
Expert Opinions on EFT for Holiday Stress
Clinicians you consult often describe EFT as a practical adjunct for acute holiday stress, noting its brief, repeatable protocol fits well into family or work routines; some therapists integrate EFT with CBT to target both emotional intensity and cognitive patterns, while integrative medicine practitioners emphasize its low-risk profile for short-term anxiety relief.
Small randomized trials and case series (sample sizes typically 20-100) report reductions in anxiety scores and physiological markers like cortisol, and neuroimaging studies suggest decreased amygdala reactivity after tapping sessions. Given these findings, many licensed therapists recommend teaching you a 5-10 minute protocol to use before stressful events, applying EFT as a self-regulation tool alongside other evidence-based strategies.
Summing up
From above you can see how using Emotional Freedom Technique (EFT) tapping provides a practical, evidence-informed method to reduce holiday stress; by tapping through specific points while naming sensations and beliefs, you lower arousal, shift perspective, and regain control over your reactions, enabling clearer choices, calmer interactions, and more enjoyment of the season.
For more information on our Emotional Freedom Technique Certification program, please visit the following link – EFT Certification Program
Additional Resourses:
The effect of the Emotional Freedom Technique (EFT) on pain and depression in cancer patients: a randomized controlled trial. Kaplan, M., Çelik, H. Support Care Cancer 33, 749 (2025).
Access Here
Going beyond Top EFT. Lessa, A., Sanz, V. J. High Energ. Phys. 2024, 107 (2024).
Access Here
The Effect of EFT Therapy on Medical Imaging Process. Akaras, Esedullah Et Al. Heliyon. 14 Pages Posted: 1 Apr 2024
Access Here